Introduction to Phosphorus Crystallization
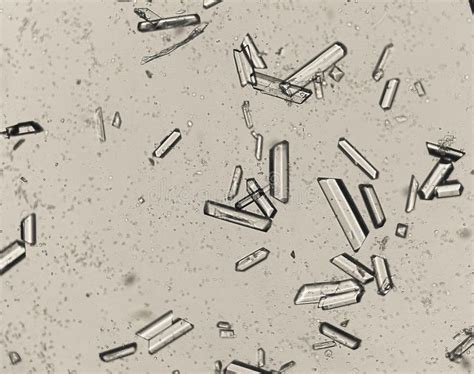
Phosphorus is a fascinating element that plays a crucial role in various fields, including chemistry, biology, and materials science. One of the most intriguing aspects of phosphorus is its ability to form crystals under specific conditions. Phosphorus crystallization is a complex process that involves the arrangement of phosphorus atoms into ordered structures, resulting in the formation of solid crystals with unique properties.
In this article, we will delve into the world of phosphorus crystal formation, exploring the underlying principles, methods, and applications of this captivating phenomenon.
The Basics of Phosphorus
Before we dive into the details of phosphorus crystallization, let’s briefly review some essential information about phosphorus itself.
| Property | Description |
|---|---|
| Symbol | P |
| Atomic number | 15 |
| Atomic mass | 30.973762 u |
| Melting point | 44.15°C (317.3 K) |
| Boiling point | 280.5°C (553.7 K) |
| Density | 1.82 g/cm³ (white) 2.69 g/cm³ (red) 2.34 g/cm³ (black) |
Phosphorus is a nonmetallic element that exists in several allotropic forms, including white, red, and black phosphorus. Each allotrope has distinct properties and crystal structures, which we will explore later in this article.
The Importance of Phosphorus Crystallization
Phosphorus crystallization is a significant process with numerous applications across various domains. Some of the key reasons why phosphorus crystallization is essential include:
-
Materials science: Phosphorus crystals exhibit unique electronic, optical, and mechanical properties, making them valuable for developing novel materials and devices.
-
Catalysis: Crystalline phosphorus compounds can act as efficient catalysts in chemical reactions, enabling the synthesis of valuable products.
-
Energy storage: Phosphorus-based crystalline materials show promise as electrode materials for high-performance batteries and supercapacitors.
-
Biomedical applications: Phosphorus crystals can be used in drug delivery systems, tissue engineering scaffolds, and biomedical imaging agents.

Allotropes of Phosphorus and Their Crystal Structures
Phosphorus exists in several allotropic forms, each with its own unique crystal structure and properties. The three main allotropes of phosphorus are:
White Phosphorus (P₄)
White phosphorus is the most reactive and unstable allotrope of phosphorus. It consists of tetrahedral P₄ molecules arranged in a cubic crystal structure. The key characteristics of white phosphorus include:
- Highly flammable and toxic
- Low melting point (44.15°C)
- Soluble in organic solvents
- Used in the production of organophosphorus compounds and smoke screens
Red Phosphorus (P~n~)
Red phosphorus is a more stable allotrope compared to white phosphorus. It is an amorphous solid that consists of polymeric chains of phosphorus atoms. The main features of red phosphorus are:
- Less reactive and toxic than white phosphorus
- Higher melting point (around 600°C)
- Insoluble in most solvents
- Used in the production of matches, fireworks, and flame retardants
Black Phosphorus (P)
Black phosphorus is the most stable and least reactive allotrope of phosphorus. It has a layered crystal structure similar to that of graphite. The notable properties of black phosphorus include:
- Semiconducting behavior with a narrow bandgap
- High charge carrier mobility
- Anisotropic thermal and electrical conductivity
- Potential applications in electronics, optoelectronics, and energy storage
Methods of Phosphorus Crystallization
There are several methods used to achieve phosphorus crystallization, each with its own advantages and limitations. The choice of method depends on the desired allotrope, crystal size, purity, and intended application. Some of the common methods of phosphorus crystallization include:
Sublimation
Sublimation is a process in which a solid substance directly transforms into a gas phase without passing through the liquid phase. In the case of phosphorus, sublimation can be used to obtain high-purity crystals of white or red phosphorus.
The sublimation process typically involves heating phosphorus in a vacuum or inert atmosphere, causing it to vaporize. The phosphorus vapor then condenses on a cooler surface, forming crystals. By controlling the temperature gradient and pressure, it is possible to achieve crystals with different sizes and morphologies.
Vapor Transport
Vapor transport is another method used for phosphorus crystallization, particularly for growing single crystals of black phosphorus. In this technique, a source material containing phosphorus is heated in a sealed tube under a temperature gradient. The phosphorus vapor generated at the hot end of the tube is transported to the cooler end, where it condenses and forms crystals.
The vapor transport process can be enhanced by using a transport agent, such as iodine or bromine, which forms volatile compounds with phosphorus and facilitates its transport. By adjusting the temperature gradient, pressure, and transport agent concentration, it is possible to control the growth rate and quality of the resulting phosphorus crystals.
Solution Growth
Solution growth is a versatile method for obtaining phosphorus crystals with well-defined sizes and shapes. This technique involves dissolving a phosphorus precursor in a suitable solvent and then inducing crystallization by altering the solution conditions, such as temperature, pH, or solvent evaporation.
One common approach is the use of organophosphorus compounds as precursors, which can be dissolved in organic solvents and then subjected to controlled hydrolysis or reduction to yield phosphorus crystals. Another approach is the use of ionic liquids as solvents, which can stabilize reactive phosphorus species and enable the growth of high-quality crystals.
Electrochemical Deposition
Electrochemical deposition is a method that utilizes electrical current to deposit phosphorus onto a conductive substrate from an electrolyte solution containing phosphorus ions. This technique allows for precise control over the thickness, morphology, and composition of the deposited phosphorus layer.
By varying the applied potential, current density, and electrolyte composition, it is possible to obtain phosphorus crystals with different allotropic forms and properties. Electrochemical deposition is particularly useful for fabricating thin films and nanostructures of phosphorus for electronic and optoelectronic applications.
Factors Affecting Phosphorus Crystallization
The process of phosphorus crystallization is influenced by several factors that determine the quality, size, and morphology of the resulting crystals. Some of the key factors include:
-
Temperature: The temperature at which phosphorus crystallization occurs plays a critical role in determining the allotropic form and crystal growth rate. Higher temperatures generally favor the formation of more stable allotropes, such as red and black phosphorus.
-
Pressure: The pressure conditions during crystallization can affect the stability and crystal structure of phosphorus. High pressures can induce phase transitions and lead to the formation of novel phosphorus allotropes with unique properties.
-
Supersaturation: The degree of supersaturation in a phosphorus-containing solution or vapor phase influences the nucleation and growth of crystals. Higher supersaturation levels promote faster crystallization rates but may also result in smaller and less perfect crystals.
-
Impurities: The presence of impurities in the phosphorus source material or crystallization environment can significantly impact the quality and properties of the resulting crystals. Impurities can act as nucleation sites, modify the crystal growth kinetics, or introduce defects into the crystal lattice.
-
Substrate: The choice of substrate on which phosphorus crystals are grown can affect their orientation, morphology, and adhesion. Substrates with compatible lattice parameters and surface chemistry can promote epitaxial growth and improve the crystalline quality of the deposited phosphorus layer.
Applications of Phosphorus Crystals
Phosphorus crystals find numerous applications in various fields due to their unique properties and versatile nature. Some of the key applications include:
Electronics and Optoelectronics
Phosphorus crystals, particularly black phosphorus, exhibit remarkable electronic and optoelectronic properties, such as high charge carrier mobility, tunable bandgap, and strong light-matter interactions. These properties make phosphorus crystals promising candidates for applications in:
- Field-effect transistors (FETs)
- Photodetectors
- Light-emitting diodes (LEDs)
- Solar cells
- Sensors
Energy Storage
Phosphorus-based crystalline materials have shown potential as electrode materials for high-performance energy storage devices, such as batteries and supercapacitors. The layered structure and high surface area of black phosphorus, for example, enable efficient ion intercalation and charge storage, leading to improved energy density and cycle stability.
Catalysis
Crystalline phosphorus compounds, such as metal phosphides and phosphates, can serve as effective catalysts for various chemical reactions. The unique electronic and structural properties of these materials can enhance the catalytic activity, selectivity, and stability, making them valuable for applications in:
- Hydrogen evolution reaction (HER)
- Oxygen evolution reaction (OER)
- CO₂ reduction
- Organic synthesis
Biomedical Applications
Phosphorus crystals have found applications in the biomedical field due to their biocompatibility, biodegradability, and ability to interact with biological systems. Some of the potential biomedical applications of phosphorus crystals include:
-
Drug delivery: Phosphorus-based nanocrystals can be used as carriers for targeted drug delivery, enabling controlled release and enhanced therapeutic efficacy.
-
Tissue engineering: Phosphorus-containing crystalline materials can serve as scaffolds for tissue regeneration, promoting cell adhesion, proliferation, and differentiation.
-
Bioimaging: Phosphorus nanocrystals with luminescent properties can be used as contrast agents for biomedical imaging techniques, such as fluorescence and photoacoustic imaging.
Challenges and Future Perspectives
Despite the significant progress made in the field of phosphorus crystallization, there are still several challenges and opportunities for future research. Some of the key challenges and future perspectives include:
-
Large-scale production: Developing scalable and cost-effective methods for the production of high-quality phosphorus crystals remains a challenge. Further optimization of existing techniques and exploration of new approaches are necessary to meet the growing demand for phosphorus-based materials.
-
Precise control over properties: Achieving precise control over the size, shape, and composition of phosphorus crystals is crucial for tailoring their properties for specific applications. Advanced characterization techniques and in situ monitoring tools are needed to gain deeper insights into the crystallization process and enable rational design of phosphorus crystals.
-
Novel allotropes and crystal structures: The discovery of new phosphorus allotropes and crystal structures with unique properties continues to be an active area of research. High-pressure synthesis, chemical modification, and computational predictions are promising avenues for exploring the rich phase space of phosphorus and identifying novel crystalline forms with desirable characteristics.
-
Hybrid and composite materials: Integrating phosphorus crystals with other materials, such as metals, semiconductors, and polymers, can lead to the development of hybrid and composite structures with enhanced functionality. Investigating the interfaces and synergistic effects between phosphorus crystals and other components is essential for realizing advanced applications in electronics, energy, and biomedicine.
-
Sustainability and environmental impact: As the demand for phosphorus-based materials grows, it is important to consider the sustainability and environmental impact of phosphorus crystallization processes. Developing green and eco-friendly synthesis methods, as well as exploring the recycling and reuse of phosphorus materials, will be crucial for ensuring the long-term viability of phosphorus-based technologies.
Frequently Asked Questions (FAQ)
-
What is phosphorus crystallization?
Phosphorus crystallization is the process by which phosphorus atoms arrange themselves into ordered structures, forming solid crystals with distinct properties and characteristics. -
What are the main allotropes of phosphorus?
The three main allotropes of phosphorus are white phosphorus (P₄), red phosphorus (P~n~), and black phosphorus (P). Each allotrope has its own unique crystal structure and properties. -
What are the common methods used for phosphorus crystallization?
Some of the common methods used for phosphorus crystallization include sublimation, vapor transport, solution growth, and electrochemical deposition. The choice of method depends on the desired allotrope, crystal size, purity, and intended application. -
What factors affect phosphorus crystallization?
The process of phosphorus crystallization is influenced by several factors, including temperature, pressure, supersaturation, impurities, and substrate. These factors determine the quality, size, and morphology of the resulting crystals. -
What are some of the key applications of phosphorus crystals?
Phosphorus crystals find numerous applications in various fields, such as electronics and optoelectronics, energy storage, catalysis, and biomedical applications. They are valued for their unique electronic, optical, and mechanical properties, as well as their biocompatibility and versatility.
Conclusion
Phosphorus crystal formation is a fascinating and complex process that has captivated researchers and engineers alike. From the basic principles of phosphorus chemistry to the advanced methods of crystallization, this article has provided a comprehensive overview of the field.
The allotropes of phosphorus, with their distinct crystal structures and properties, offer a wide range of possibilities for materials science and technology. The various methods of phosphorus crystallization, including sublimation, vapor transport, solution growth, and electrochemical deposition, enable the production of high-quality crystals for diverse applications.
As we continue to explore the potential of phosphorus crystals in electronics, energy storage, catalysis, and biomedicine, it is clear that this field holds immense promise for the future. However, challenges remain in terms of large-scale production, precise control over properties, and the discovery of novel allotropes and crystal structures.
By addressing these challenges and embracing the opportunities for innovation, researchers and engineers can unlock the full potential of phosphorus crystallization and contribute to the development of advanced materials and technologies that will shape our world in the years to come.




Leave a Reply